Where Does the Body’s Vitality Come From?

For more than a century, scientists have noticed water has a variety of unusual properties that do not fit within the classic model of it being just a solid, liquid or gas.
Gerald Pollack built upon those observations, and eventually determined that when a negatively charged surface is present, if an appropriate energy source is also present (particularly infrared — which is everywhere), the water will assemble itself into layers of offset hexagonal sheets with the formula H3O2 that behave like a liquid crystal.
Note: In addition to light, sound can also generate H3O2.
In my eyes, liquid crystalline water is a critically under appreciated area of science as it provides a mechanism to explain many of the unexplained mysteries of the body (e.g., how do so many fluids flow within it despite there being no known pump to move them) and a way to put words into much of what we frequently observe within the body.
What Is Liquid Crystalline Water?
Liquid crystalline water has a significant degree of solidity, and will expel most things from entering it (e.g., polystyrene microspheres), including the displaced hydrogen atoms (as it is H1.5O not H2O). These displaced positively charged H atoms (henceforth referred to as protons), in turn assemble immediately outside this lattice, thereby creating a pH and charge gradient which can be measured.
In many cases, these H3O2 lattices can be enormous — in favorable conditions, Pollack and others have measured ones ranging from 0.1 millimeters to 0.5 millimeters (100,000 to 500,000 nanometers or nm) in size. Cells depend on this water, so they contain a large number of surfaces from which the water can form.
For example, 20% of the cell is occupied by its cytoskeleton (a protein lattice which maintains its structure), and analysis of high-voltage electron micrographs have shown that within the cytoskeleton, over half of the water present is within 5 nm of a surface it could potentially form H3O2 on (for reference a single H2O molecule is 0.27 nm in size).
Since the surface sites that water can structure on are so closely packed together in cells, it is understandable why liquid crystalline water (also commonly called EZ water) would be so much more apparent to individuals observing living cells under a microscope (which in fact is where much of the early research on H3O2 originated from). This in turn raises another question: why are cells designed to create so much liquid crystalline water?
Note: A longer article describing the long history of research into liquid crystalline water (H3O2) and its physical properties can be read here.
Mysteries of the Cell
In the previous article, I discussed a common issue I observe within science. When an incorrect model is utilized to explain a natural process, discrepancies between the model’s predictions and reality will inevitably appear.
One would expect that when this happens, it would encourage those espousing the erroneous model to re-examine their model, but instead, since so much has been invested into that model, they instead will denounce any challenges to it, and come up with innumerable creative ways to explain away each failure of the existing model.
Consider, for example, the initial promises of the vaccines (if you get two doses, you were told that you would be completely immune, transmission would stop, and COVID-19 would rapidly fade into memory). Since the clinical trials for the vaccines were fraudulent, none of the vaccines’ promises materialized, and the COVID situation instead became worse.
However, instead of healthcare authorities (and the medical community) admitting their mistake and switching to a different approach for managing COVID-19, they doubled down on the vaccine mandates and moved the goal posts over and over in regards to what mass-vaccination (and boosting) was actually supposed to accomplish.
Likewise, for cellular biology, our knowledge of the cell is surprisingly primitive and the existing models often fail to explain what occurs within the cellular environment. However, since the alternative models are not generally accepted by the scientific community, we have been forced to continually patch the existing models so that they can somewhat account for the innumerable mysteries of life.
At this point, I believe one of the key causes of this situation is scientific research becoming distorted to prioritize focusing on discoveries that industry can profit from.
For example, immunology has a narrow focus on the aspects of the immune system which can be targeted by vaccination or proprietary drugs, which in turn has left many other components of the immune response neglected (the immune system is commonly referred to as one of the least understood systems in the body).
Similarly, since pharmaceutical drugs often work by affecting receptors and channels in cells, cellular biology has adopted a narrow-minded focus on just those aspects of a cell.
In turn, the liquid crystalline phase of water provides a variety of explanations for many of the biological phenomena that the existing models simply do not adequately address. In this article, I will focus upon a few of them.
Note: Much of what is discussed in this article is covered in more detail within these three books (similarly, the majority of the references for this article are sourced from these books, so I will not repetitively cite them throughout the article). If you wish to further study the subject yourself, I would recommend reading those books (all authored by Gerald H. Pollack) in this order:
- The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor (2013)
- Cells, Gels and the Engines of Life: A New, Unifying Approach to Cell Function (2001)
- Phase Transitions in Cell Biology (2008) — this is the most technical of the three
Cellular Integrity
One of the major puzzles of biology is the immense durability that cells have. If you consider the classic model — cells being bags of liquid contained within a fluid mosaic membrane, it should be effortless for external forces to “pop” cells and have all of their contents spill out. Yet in most cases, cells maintain their integrity despite significant stressors.
Cells can survive traumas, including being guillotined in half, drawn and quartered (so specific components can be isolated and worked with — such as when performing in vitro fertilization), or shot full of holes with electrical bullets, each of which one would expect would be sufficient to “pop” them. However, in each instance, cellular integrity of the remaining component persists.
Similarly, if the membrane from a cell is removed, its internal contents remain in place rather than rapidly dispersing. It has also been known for over 50 years that if muscle fibers lose their membranes, their functional ability (creating a contractile force) remains intact.
Three clues help to explain these phenomena. The first is that — as Pollack has shown — water droplets have a remarkable amount of integrity, and like cells, will maintain their integrity (and subsequently fuse back together) if a micro-blade is used to guillotine them in half. The second is that Pollack has also shown water droplets contain a significant degree of liquid crystalline water which likely is what confers their integrity.
The third is that the water molecules in cells predominantly exist within gels, which are composed of that same liquid crystalline water. Put differently, this means that a cell’s stability is largely a property of its water holding it together rather than the external structure which encapsulates it.
Another important aspect of cellular architecture should now be considered. As the conditions for liquid crystalline water formation are present throughout the cell (negatively charged hydrophilic surfaces and ambient infrared energy), the cells should rapidly be filling themselves with liquid crystalline water layers hundreds of micrometers in thickness.
Yet, throughout the cells, the surfaces are often only fractions of a micrometer apart. This means that the structure of the cell depends upon the continual formation of liquid crystalline water, but simultaneously constrains that crystalline structure from growing to its full size.
Tensegrity
Classically in architecture, buildings are created by having strong skeletons upon which the rest of the structure rests. For example, in the old days, we frequently used stone pillars; nowadays, skyscrapers require steel superstructures to meet the needs of these buildings:

This model often does not work within living organisms, because life, unlike those buildings, requires rapid movement, and living organisms simply cannot produce solid structures with the same strength as steel beams. However, an alternative and more complex architectural model has been developed which is frequently utilized by those who embrace complexity within their models.
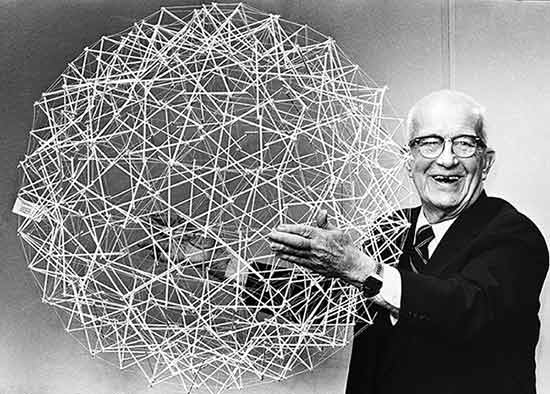
Tensegrity (short for tensional integrity), was a model first put forward by Buckminster Fuller. It posits that if a series of non-compressible structures are linked together by a lattice of elastic connections (which can store tension when stretched), a much stronger structure is created.
This is because any force the structure receives will be equally distributed through each of those elastic connections rather than concentrating on a single component (e.g., the stone pillar), and thus, much less likely to exceed the breaking point of any single structural component.
Fuller’s work subsequently inspired numerous buildings to be built on the principles of tensegrity. This is a classic picture of him holding a tensegrity sphere he made:
Biotensegrity encapsulates the realization that this same system also occurs throughout nature. For the human body, at each level of organization, a linked tensile matrix is present that confers its stability.
One French hand surgeon, Jean-Claude Guimberteau, has likewise done a remarkable job of demonstrating the presence of linked tensile structures at the level of fascia (a connective tissue present throughout the body many manual therapists work with), through small (magnified) cameras placed in the body during minimally invasive surgeries:
Note: At the magnification scale used here and in his other videos, liquid crystalline water can be directly observed coating these structures. Also note its lubricating quality that allows the structures to slide past each other. Loss of this lubrication appears to create a variety of issues within the body.
As the years go by, there appears to be a greater consensus within the holistic medical field that biotensegrity is a valid model for understanding the body, and that linked networks of tension are present from the largest to the smallest levels of the body (e.g., the cytoskeleton is the elastic connecting unit within each cell). However, while this theory is frequently discussed, there are still two major unaddressed issues with it, which I believe liquid crystalline water can explain.
The first is that in Guimberteau’s work, he had observed that tiny vacuoles (enclosed compartments filled with water) throughout the body form the basic incompressible unit that much of the body’s tensegrity depends upon.
The second is that at an even higher magnification within cells, while a cytoskeleton is present that is under tension, there is nothing that can be seen which creates that tension (and it is highly debatable if the cell’s connections to the extracellular matrix suffice to create that tension). This matters because the structural strength of a tensegrity system only emerges when a structure’s components are under tension.
In the case of the former, I believe that the microvacuoles are filled with liquid crystalline water (which cannot be compressed). In the case of water droplets, Pollack concluded that the mutual repulsion between positively charged protons (pushed to the center of the droplet by sheets of H3O2) creates an outwards force resisted by the liquid crystalline boundary of the droplet.
The balance between these two forces results in the droplet assuming a spherical form, and I believe the same mechanism is at work in the microvacuoles throughout the human body.
In the case of the latter, it is important to remember how much expansion is produced by proteins that create gels (many gels are over 99.9% water). Pollack, in turn, has shown that the liquid crystalline water that is constantly trying to form within the cell is unable to reach its full size due to proteins resisting the stretch that would have to occur were the liquid crystalline gel to grow to its full size.
This happens both at the level of the cell (as the cytoskeleton stretches as the cell expands to its maximum size, until it cannot allow further expansion to occur) and within proteins throughout the cell.
At the protein level, the body often relies upon chemical bonds to be made between proteins to constrain the maximum expansion that can occur in response to a gel forming. Additionally, proteins can alternate between a folded and an unfolded conformation (e.g., a helix vs. a coil), something often determined by the tension applied to the protein (such as the expansive pressure of liquid crystalline water within a gel).
There are a variety of importance consequences of this conformation change which will be discussed later in this article (e.g., for the physiology of muscles). Additionally, many other components of the body also appear to rely upon liquid crystalline water:
“Studying collagen, Melacini et al. noted the importance of water in stabilizing its triple helix crystalline structure. They found that water within the collagen helix forms a “semi-clathrate-like structure that surrounds and interconnects triple helices in the crystal lattice.”
Water within the matrix of bone is likewise highly structured, a structuring that has been found associated not only with organic macromolecules such as collagen and proteoglycans, but with the mineral surfaces as well. Water, in fact, seems to play a foundational role in orienting mineral nanoparticles into parallel arrangements within the bone matrix, providing this orientation even in the absence of organic molecules.”
Everyday Gel Expansions
Many technologies we are familiar with (e.g., diapers) rely upon hydrogels which can expand into a semisolid structure which retain water. Similarly, we can directly observe that many larger processes within the body are also dependent upon water’s tendency to assemble into the larger liquid crystalline structures.
Because the normal architecture of protein’s cross-linking behavior limits how much gels can expand (as the proteins which would need to separate to accommodate the growing gel are prevented from doing so by cross links between them), when tissue or protein is damaged, this limitation can be partially removed. As a result, it can be seen on a microscopic scale that human tissue will swell and expand when it is damaged.
Likewise, Pollack has argued that this is most likely what happens when you experience a musculoskeletal trauma. In Pollack’s proposed model, the initial gel formation further expands the existing tear, and this progressive expansion of liquid crystalline water eventually leads to visible swelling.
On a larger scale, one of the major engineering challenges for the body is having weight-bearing joints like the knees be able to maintain their range of motion without becoming damaged by the continual friction they experience on a daily basis.
A remarkable characteristic about liquid crystalline water is that, provided the surface from which it forms remains intact, liquid crystalline water can be destroyed and then reform, along with its almost frictionless surface, over and over again.
Because of this, the liquid crystalline water ends up being the component which absorbs the stress experienced by healthy joints, and provided the joint is healthy, this water can instantly regenerate from that stress. In conjunction with this regenerating layer of negatively charged liquid crystalline water, at the very center of the joint, there is a pocket of positively charged protons which repel from each other and create an expansive pressure (like what is seen in a water droplet).
Since the joint capsule seals this region, those protons are unable to escape and thus effectively function like repelling magnets (e.g., consider a maglev train) that resist the weight of the body and maintain the central space within the joint.
One of the things I consider the most compelling about Pollack’s model for the joints is the specific quality of synovial fluid inside the knee joint. When you view it on a camera during an arthroscopic surgery, diffusion within it is visibly slowed, while if you directly extract it (e.g., during a knee aspiration), you can tell that it has a much thicker and gelatinous quality, something I have learned to associate with the presence of liquid crystalline water.
Pollack likewise argues that his model suggests EZ (liquid crystalline) water’s behavior resembles that of a gelatinous egg white which is semisolid when left alone, yet able to flow in response to an imposed shear force.
Cellular Gradients
Note: A “gradient” describes a difference in the concentration of things in two different areas. This could include electrical charges (i.e. batteries depend upon gradients), electrolytes, or temperatures — and many gradients provide an easily harvestable source of energy.
The existing paradigm of physics asserts the following:
- The natural state of things is to be disordered and evenly mixed.
- Anytime you make something become more ordered (e.g., forming a crystal or creating a gradient between two areas), energy must be expended to create that ordering.
- When an ordered structure becomes disordered, energy is released in the process which can sometimes be harvested (e.g., combusting wood in a wood stove to heat our homes).
- Anytime energy is released from something, if you attempt to capture and store that energy, some energy will always be lost.
These laws, in turn, are used to refute the possibility that any type of “free-energy” system can exist, such as the exclusion zone (EZ) water system Pollack has proposed where numerous harvestable gradients are created. Unfortunately for the existing paradigm, biology often appears to violate these laws, as it is continually moving towards a more ordered state rather than the disordered state the current paradigm predicts.
The current resolution for this paradox (which won a Nobel prize) is that living organisms function as “dissipative structures,” which exchange the order in large amounts of ordered components they accumulate from their environment in return for imparting order to their own disordered contents.
Although to some extent this allows the existing paradigm to sustain itself, I do not believe it is entirely accurate as water, through its liquid crystalline structure, has the ability to store radiant energy that passes through it and convert that energy to order the body can then utilize.
The Sodium Potassium Gradient
One of the things living cells are well known for doing is concentrating potassium inside themselves and reciprocally expelling sodium. Since the concentration inside and outside cells differs, a gradient exists, which, by the existing laws of physics should try to equalize itself and rapidly disappear.
Since this does not happen, the existing model has argued that the cellular membrane prevents the passage of most but not all sodium and potassium (thereby inhibiting the gradient from equalizing itself) and that sodium potassium pumps on the cellular membrane exist which continual swap sodium inside the cell for potassium outside the cell.
Because this exchange is so vital to maintaining the health of the cell, a large focus in cellular biology is placed on the importance of the sodium potassium exchange pump. Unfortunately, there are three fundamental problems with this model (the evidence of each of these and more is presented by Gilbert Ling here):
- The math does not add up — the existing sodium potassium pumps simply do not have the capacity to counteract the natural undoing of the sodium and potassium gradients. For example, in muscle cells, maintaining the sodium potassium gradient with pumps requires between 4 to 30 times the total available energy in the cell.
- Cells are able to maintain a gradient with a variety of other undesirable components they expel (e.g., bacteria expelling antibiotics) and to explain these phenomena, more and more pumps are identified to try to support the model. This is a problem because it is unlikely that cells have the capacity to simultaneously sustain so many different pumps.
- Ling took frog muscle cells whose environment was altered so that they were completely starved of energy (which is needed to operate the sodium potassium pump). Despite this, the gradient was maintained.
This then begs the question..